MOBIO Member Highlight
Benson Hill: Nourishing Innovation with Advanced Genetics and Technology
Benson Hill is an ag tech company on a mission to lead the pace of innovation in soy protein through differentiated and advantaged genetics. Leveraging downstream insights and demand, Benson Hill utilizes its CropOS® technology platform to design and deliver food and feed that’s better from the beginning: more nutritious, and more functional, while enabling efficient production and delivering novel sustainability benefits to food and feed customers.
Situated in the 39 North Ag Tech Innovation District, Benson Hill is headquartered in St. Louis, where most of its research and development activities are managed.

In a strategic move towards an asset-light business model, Benson Hill recently divested its soy-crushing and food-grade manufacturing operations, opting to focus on its core strengths in research and development. This strategic shift allows the company to participate across the value chain through efficient partnerships while maintaining its ability to drive seed innovation.
Leadership believe that moving to an asset-light business model will enable the organization to focus on research and development competitive advantages while participating across the value chain through partnerships that are more efficient to scale acreage, require less operating expense and are more capital-efficient. This model maintains the company’s ability to solve end-user challenges with seed innovation.
The company has identified three opportunities to monetize Benson Hill’s technology. First, by licensing germplasm to seed companies. Second, by direct seed and grain sales to farmers. And third, through technology access fees and value-based royalties from seed companies, processors and end users. In the future, Benson Hill plans to enter the animal feed market and secure partnerships and licensing agreements to scale its product offerings.
The emergence of significant market headwinds in the food, aquaculture, and specialty oil markets is a factor in the decision to reshape the business to best position Benson Hill’s proprietary product portfolio and future product pipeline for significant growth.
Recent advances in its soybean breeding program will drive significant expansion of Benson Hill’s seed portfolio by 2025. The latest field evaluations on the third generation of Ultra High Protein Low Oligosaccharides (“UHP-LO”), non-GMO soybean varieties showed protein gains of two percent over the previous generation and achieved a yield gap of only three to five bushels per acre, compared with commodity GMO soybeans.
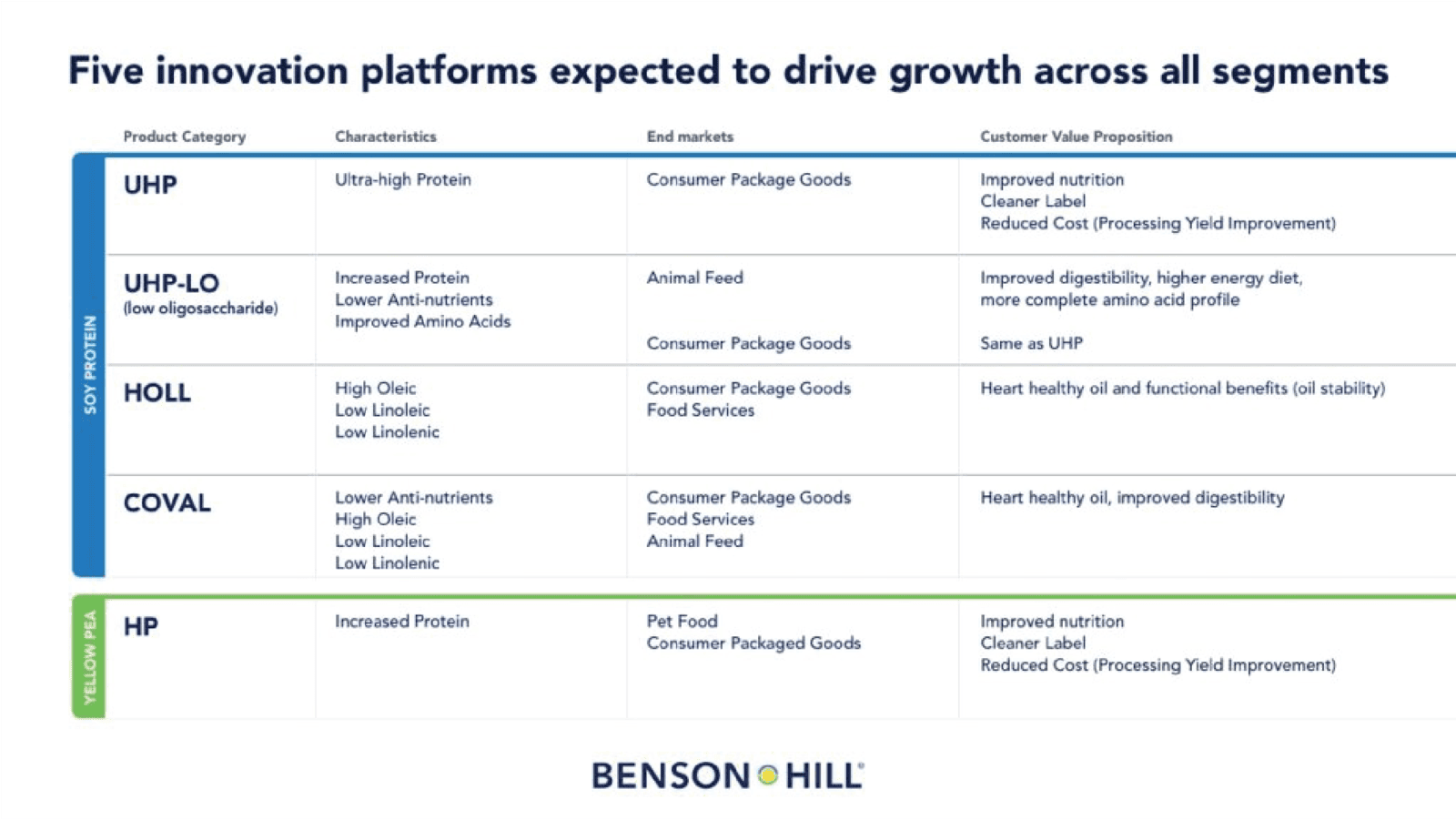
Benson Hill’s herbicide-tolerant Ultra High Protein (“UHP”) soybean varieties are on track for commercial release in 2025, with acreage and further portfolio expansion expected in 2026. This is a major step in providing farmers with options for weed control and enabling lower-cost, broadacre production of already advantaged UHP soybeans for the animal feed industry.
Evolving Food and Feed Industry
By combining proprietary data with artificial intelligence (“AI”) capabilities, coupled with advances in plant genomics and an asset-light strategy, Benson Hill believes it can accelerate the development and delivery of better food and feed options from the beginning—with a focus on both quantity and quality. Genomics has been used for decades to develop crops for the food and feed system, but most agricultural companies have focused almost exclusively on increasing the yield of a few crops, resulting in commodity ingredients. While focus on quantity is important, it often comes with trade-offs in areas such as nutrient density.
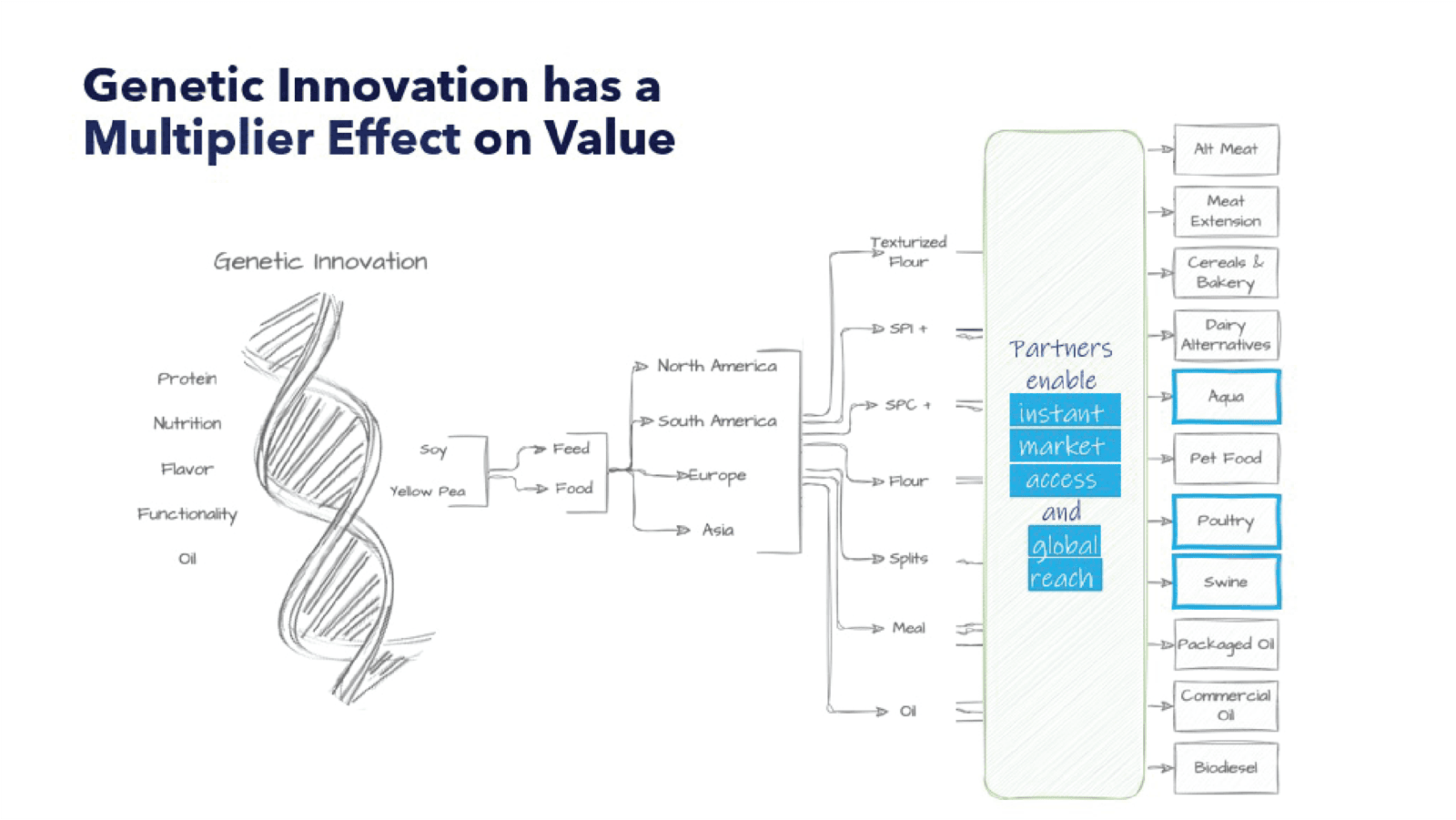
By leveraging deep insights on its proprietary soybean germplasm, Benson Hill is strategically positioned to drive seed innovation in broadacre opportunities for the aquaculture, pet food, swine and poultry markets – some 90 percent of the soy market. These steps will allow for speed to market with solution-based products, giving the company the opportunity to recommoditize soybeans for animal feed and food.
For more information about Benson Hill and its transformative work in seed innovation, visit www.bensonhill.com.














